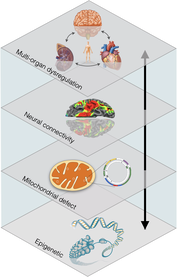
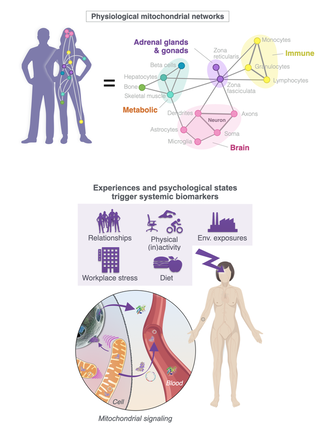
Mitochondrial Psychobiology is the study of the interactions between psychological states and the biological processes that take place within mitochondria. It also examines how mitochondrial biology influences neural, endocrine, and immune systems known to transduce psychological experiences into health outcomes.
Ongoing projects
|
We are running the Mitochondrial Stress, Brain Imaging, and Epigenetics (MiSBIE) study to understand how (ab)normal mitochondrial phenotypes interact with cellular function, neural circuits, and psychosocial functioning among individuals with rare mtDNA defects.
MiSBIE combines deep mitochondrial and cellular profiling of circulating immune cells, psychosocial and neuropsychological assessments, genetic and epigenetic analyses, clinical assessments of disease severity, multi-system physiological (dys)regulation, diurnal profiling of neuroendocrine rhythms and sleep, structural and functional neuroimaging, among other outcome measures. This project will allow testing new hypotheses linking the mind and mitochondria, including the bioenergetic basis of psychiatric symptoms, the source of resilience, and the role of hypermetabolism in mitochondrial diseases. The clinical portion of MiSBIE led by Dr. Michio Hirano may lead to new insights into brain-body processes that may influence mitochondrial disease progression, representing an opportunity to have a positive impact on the lives of patients. MiSBIE brochure In the complimentary Stress Health and Emotion Survey (SHES), we ask patients with mitochondrial diseases whether they experience a connection between how their emotions and specific clinical symptoms. This research theme also will generate cellular-level insight into the basis of chronic stress pathophysiology, allostasis, and allostasic load. |
- Cellular allostatic load is linked to increased energy expenditure and accelerated biological aging
Bobba-Alves N, Sturm G, Lin J, Ware SA, Karan KR, Monzel AS, Bris C, Procaccio V, Lenaers G, Higgins-Chen A, Levine M, Horvath S, Santhanam BS, Kaufman BA, Hirano M, Epel E, Picard M. Psychoneuroendocrinology 2023 Link PDF - OxPhos defects cause hypermetabolism and reduce lifespan in cells and patients with mitochondrial diseases
Sturm G, Karan KR, Monzel AS, Santhanam BS, Taivassalo T, Bris C, Duplaga SA, Cross M, Towheed A, Higgins-Chen A, McManus MJ, Cardenas A, Lin J, Epel ES, Rahman S, Vissing V, Grassi B, Levine M, Horvath S, Haller RG, Lanaers G, Wallace DC, Tavazoie S, Procaccio V, Kaufman BA, Seifert EL, Hirano H, Picard M. Commun Biol 2023 Link PDF
- Mitochondrial respiratory chain function modulates LPS-induced inflammatory signatures in human blood
Karan KR, Trumpff C, McGill MA, Thomas JE, Sturm G, Lauriola V, Sloan RP, Rohleder N, Kaufman BK, Marsland AL, Picard M.
Brain Behav Immun-Health 2020 PubMed PDF Suppl
- An energetic view of stress: Focus on mitochondria
Picard M, McEwen BS, Epel ES, Sandi C. Front Neuroendocrinol 2018 PubMed PDF
- Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory and transcriptional responses to
psychological stress
Picard M, McManus MJ, Gray J, Nasca C, Moffat C, Kopinsky P, Seifert E, McEwen BS, Wallace DC. PNAS 2015 PubMed PDF
|
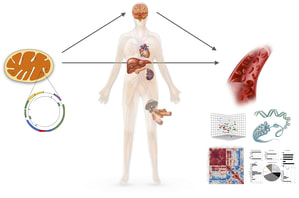
Living organisms are bound to age. But what determines the rate at which they age and functionally decline? We explore the link between mitochondrial energy transformation and signaling with (cellular) time perception and the aging process.
We study aging at two main levels: in cells, and in people. The Cellular Lifespan Study is an in vitro longitudinal, Hayflick-style, multi-omics analysis of time-dependent changes occurring across the lifespan of primary human fibroblasts. The dataset includes a rich set of measurements based on DNA, RNA, secreted proteins, cellular phenotypes, and bioenergetics. See Resources for details. The MiSBIE study also includes a rich set of aging-related biological, functional, and psychosocial measures to extend and validate our in vitro findings. To examine the psychobiology of aging in humans, we also developed a hair pigmentation pattern (HPP) method to quantify hair greying and reversal. Using hair length as tree rings in the Stress, Mitochondria and Aging Hair (SMAH) study we quantitatively showed with our collaborator Ralf Paus (U Miami) that human heir greying is reversible and linked to acute stressful events. |
- Cellular allostatic load is linked to increased energy expenditure and accelerated biological aging
Bobba-Alves N, Sturm G, Lin J, Ware SA, Karan KR, Monzel AS, Bris C, Procaccio V, Lenaers G, Higgins-Chen A, Levine M, Horvath S, Santhanam BS, Kaufman BA, Hirano M, Epel E, Picard M. Psychoneuroendocrinology 2023 Link PDF
- A multi-omic and bioenergetics longitudinal aging dataset in primary human fibroblasts with mitochondrial perturbations
Sturm G, Monzel AS, Karan KR, Michelson J, Ware SA, Cardenas A, Lin J, Bris C, Santhanam B, Murphy MP, Levine ME, Horvath S,
Belsky D, Wang S, Procaccio V, Kaufman BA, Hirano M, Picard M. Sci Data 2022 Link PDF
- Quantitative mapping of human hair graying and reversal in relation to life stress
Rosenberg A, Rausser S, Ren J, Mosharov EV, Sturm G, Ogden RT, Patel P, Soni RK, Lacefield C, Tobin DJ, Paus R, Picard M.
eLife 2021 PubMed PDF eLife Digest
|
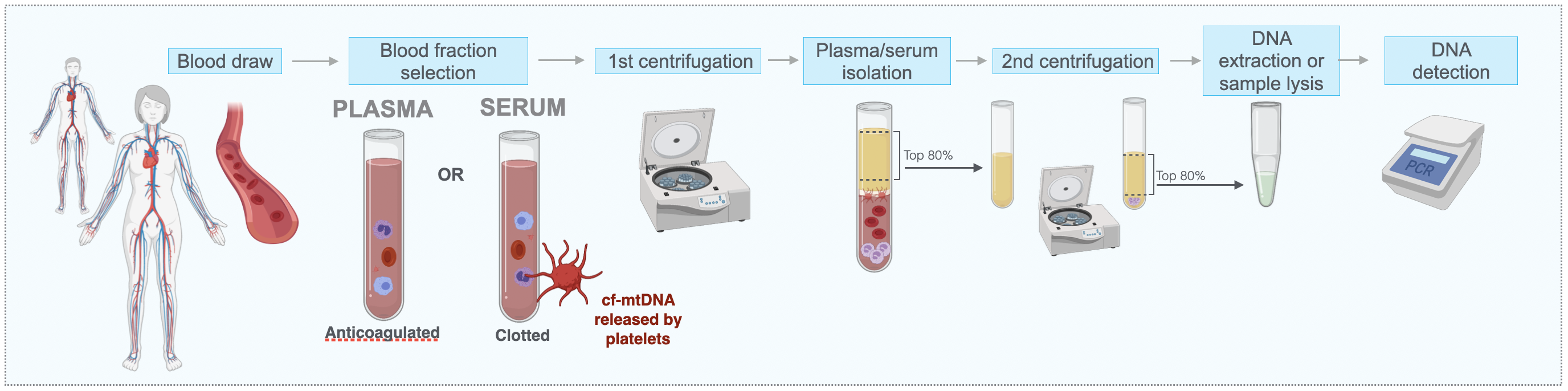
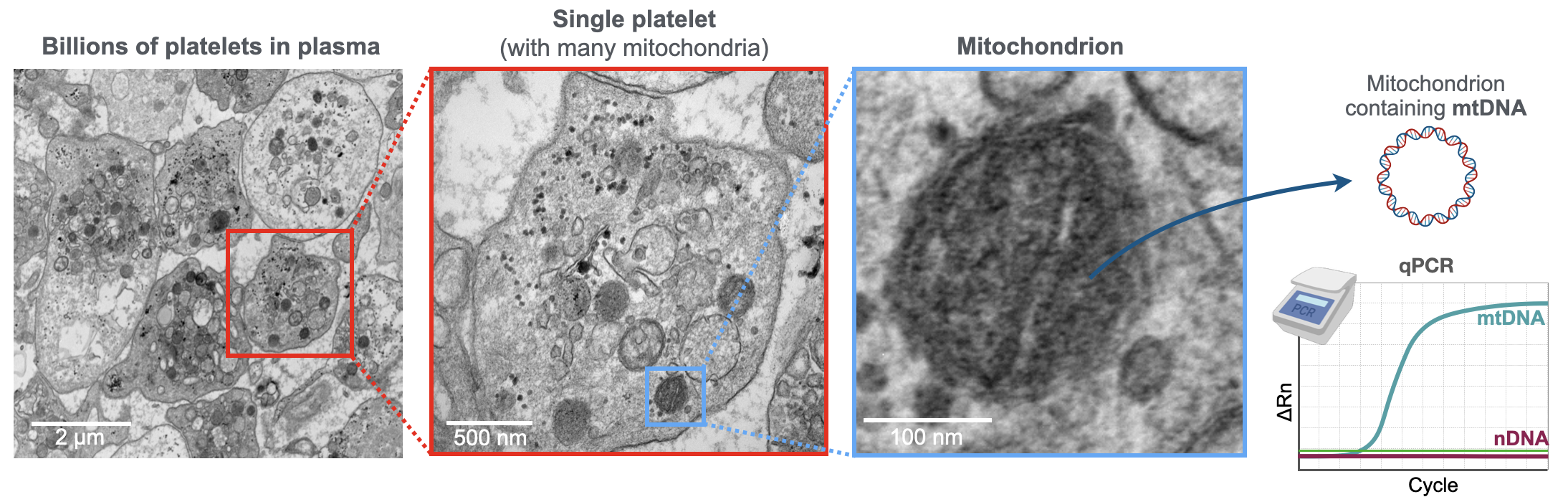
Mitochondria can release their genome extracellularly, where it is detectable as cell-free mitochondrial DNA (mtDNA). cf-mtDNA levels vary dynamically in blood and other human biofluids, including in saliva.
In the Mitochondria and Psychological Stress (MaPS) study with our collaborators Anna Marsland and Brett Kaufman (Pittsburgh) and in MiSBIE, we map the kinetics of blood cf-mtDNA responses to challenges. We develop new methods to selectively quantify cf-mtDNA dynamics in biofluids. In the Saliva Mitochondria Study (SMS) we generate hourly diurnal profiles of saliva cf-mtDNA in healthy women and men to understand the drivers of cf-mtDNA dynamics. Through in vitro models we also examine how cf-mtDNA is induced by mitochondrial stress and with aging. Establishing the triggers, modes of transport, and targets of cf-mtDNA will fill in important knowledge gaps around mitochondrial psychobiology in humans. In the Mitochondrial Daily Energy Expenditure (MDEE) study we examine cf-mtDNA and energy expenditure along the day-night cycle, including during sleep when consciousness leave the body, and when the organism shifts to an anabolic, restorative bioenergetic state. MDEE Brochure |
- MitoQuicLy: A high-throughput method for quantifying cell-free DNA from human plasma, serum, and saliva
Michelson J, Rausser S, Peng A, Yu T, Sturm G, Trumpff C, Kaufman BA, Rai AJ, Picard M. Mitochondrion 2023 Link PDF
- Dynamic behavior of cell-free mitochondrial DNA in human saliva
Trumpff C, Rausser S, Haahr R, Karan KR, Gouspillou G, Putterman E, Kirschbaum C, Picard M. Psychoneuroendocrinology 2022 Link PDF
- Stress and circulating cell-free mitochondrial DNA: a systematic review of human studies, physiological considerations, and technical recommendations
Trumpff C, Michelson J, Lagranha CJ, Taleon V, Karan K, Sturm G, Lindqvist D, Fernström J, Moser D, Kaufman BA, Picard M.
Mitochondrion 2019 PubMed PDF Protocol
- Acute psychological stress increases serum circulating cell-free mitochondrial DNA
Trumpff C, Marsland AL, Basualto C, Martin JL, Carroll JE, Sturm G, Gu Z, Vincent A, Kaufman BA, Picard M. Psychoneuroendocrinol
2019 PubMed PDF
|
|
Without energy flow, the human brain is an inanimate fatty blob. But powered by energy, the brain is an amazing signal integration center that allow us to think, feel, love, interact with others and with the world around us.
In MiSBIE, with our collaborators Tor Wager (Dartmouth) and Michel Thiebaut de Schotten (Bordeaux), we examine brain function and structure in individuals with rare mtDNA diseases, to understand the effects of energetic constraints on large-scale brain networks and connectivity. Our work with Phil De Jager and colleagues at the CTCN examines fundamental aspects of mitochondrial biology in the aging human brain, and how psychosocial exposures interact with brain mitochondria. This builds on mitochondrial phenotyping in hundreds of human brains in the MitoBrain ROSMAP project. In mice, we have begun interrogating regional variation and network organization of mitochondrial phenotypes across the brain. In collaboration with Chris Anacker and Dani Dumitriu (Columbia), Manish Saggar (Stanford), and Carmen Sandi (EPFL), we have also explored the link between mitochondrial energetics and anxiety-related behavior. To bring mitochondrial science to human neuroscience, together with investigators in the MIND division at NYSPI, Eugene Mosharov and the Sulzer lab, and Michel Thiebaut de Schotten (Bordeaux) we are building the MitoBrainMap v1.0 - developing hardware/software approaches to physically voxelize frozen human brain sections at fMRI resolution. Deploying our mitochondrial phenotyping platform to hundreds of human brain voxels will allow to build the first systematic map of mitochondrial bioenergetics in the human brain. |
- Brain mitochondrial diversity and network organization predict anxiety-like behavior in mice.
Rosenberg A, Saggar M, Monzel AS, Devine J, Rogu P, Mosharov EV, Junker A, Sandi C, Dumitriu D, Anacker C, Picard M. Nat Commun 2023 Link PDF
- Mitochondrial respiratory chain protein co-regulation in the human brain
Trumpff C, Owusu-Ansah E, Klein H, Lee A, Petyuk V, Wingo TS, Wingo AP, Thambisetty M, Ferrucci L, Seyfried NT, Bennett DA, De Jager PL, Picard M. Heliyon 2022 Link PDF
- Mitochondrial DNA quantity and quality in the human aged and Alzheimer’s disease brain.
Klein H, Trumpff C, Yang HS, Lee AJ, Picard M, Bennett DA, De Jager. Mol Neurodegener 2021 PubMed PDF
|
We build new laboratory methods, sample collection and processing procedures, and computational pipelines to serve the developing field of mitochondrial psychobiology.
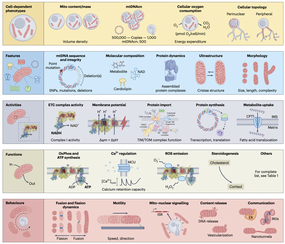
Work around this theme contributes to enhance and enable new lines of inquiry within our laboratory and others. This includes development of a mitochondrial phenotyping platform where we can quantify the enzymatic activities of respiratory chain complexes in 96-well format, mapping mitochondrial phenotypes in purified immune cells and cell mixtures, developing a frozen human brain voxelization approach, optimizing cell-free mtDNA quantification methods for biofluids, and perfecting longitudinal designs in people and in cells to capture health dynamics from organelle to organism. Our work on mitotyping examines the molecular diversity of mitochondrial phenotypes (Mitotypes) from omics data aross the human body, at single-cell resolution. |
- Multifaceted mitochondria: Moving mitochondrial science beyond function and dysfunction
Monzel AS, Enriques JA, Picard M. Nat Metab 2023 Link PDF
- Dynamic behavior of cell-free mitochondrial DNA in human saliva
Trumpff C, Rausser S, Haahr R, Karan KR, Gouspillou G, Putterman E, Kirschbaum C, Picard M. Psychoneuroendocrinology
2022 Link PDF
- Mitochondrial phenotypes in purified human immune cell subtypes and cell mixtures
Rausser S, Trumpff C, McGill MA, Junker A, Wang W, Ho S, Mitchell A, Karan K, Monk C, Segerstrom S, Reed R, Picard M.
eLife 2021 PubMed PDF
- 3D neuronal mitochondrial morphology in axons, dendrites, and cell bodies of the aging mouse hippocampus
Faitg J, Lacefied C, Davey T, White K, Laws R, Kosmidi S, Reeve AK, Kandel E, Vincent AE, Picard M. Cell Rep 2021 PubMed PDF - Blood mtDNA copy number: What are we counting?
Picard M. Mitochondrion 2021 PubMed PDF
|
|
"Clearly, an understanding of the simple is necessary to understand the more complex, but whether it is sufficient is questionable." |